
Surface Tension…Critical Micelle Concentration…
Terminology You Should Know for Formulation Success
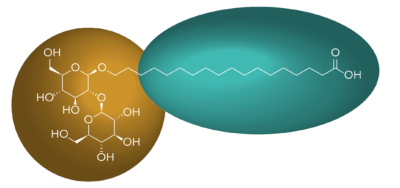
Figure 1: Structure of Locus PI’s Linear Stearic Sophorolipid. Hydrophilic regions are highlighted in blue and lipophilic regions are highlighted in green.
Formulators use surfactants, and now biosurfactants, as emulsifiers, solubilizers, cleansers, wetters and foamers, but how exactly do they work? Formulation success can be amplified by understanding the action of these ingredients and three key terms…
- Surface tension
- Micelles
- Critical micelle concentration
Surface Tension
Water, due to its strong hydrogen bonding, has extremely high surface tension. This is why water beads into droplets and puddles when spilled on a surface, instead of creating a thin uniform layer of fluid. The activity of surfactants and biosurfactants is based on their ability to disrupt the hydrogen bonding of water by grouping at the surface of the air-
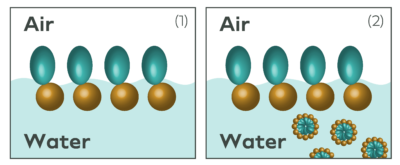
Figure 2: Surfactants in water. (1) shows surfactants orienting at the air-water interface. (2) shows surfactants forming micelles as the concentration of the surfactant exceeds the CMC.
water interface (see where the name comes from?). At this interface, the polar regions of a surfactant molecule will interact with polar water molecules and the nonpolar region of a surfactant are repelled out of the solution towards air.
Micelles
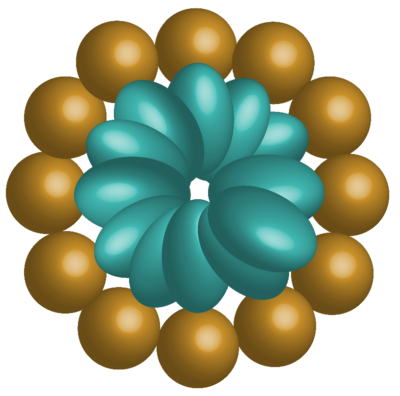
Figure 3: Micelle structure in water showing hydrophilic regions of a surfactant on the exterior and lipophilic regions on the interior.
When this interface is saturated with surfactant or biosurfactant molecules, any additional molecules added to the solution will form specialized structures called micelles. Micelles are structures that resemble cell membranes—microscopic spheres where surfactants are orientated such that the outside surface is composed of the polar regions and the interior is composed of the nonpolar regions.
Critical Micelle Concentration
The concentration at which micelles are formed is called the Critical Micelle Concentration (CMC). The CMC also describes the concentration that achieves the greatest surface tension reduction of a surfactant. As micelles only form when the air-water interface is saturated with surfactants, it is not possible for the surface tension to be reduced further past the CMC.
The critical micelle concentration is important for formulators as it is the minimal concentration of the surfactant or biosurfactant needed to achieve maximum surface tension reduction performance.
After achieving the critical micelle concentration, any additional surfactants or biosurfactants in a formulation will form micelles. Micellar water, a relatively new personal care product, is formulated by adding surfactants in large excess to the CMC. These products are used to gently remove make-up, dirt and oils from sensitive areas such as the face. As micellar water grows in popularity, formulators are looking for bio-based ingredients to replace surfactants and offer a more sustainable and natural alternative.
Importance of Critical Micelle Concentration and Surface Tension Reduction in Product Formulations
Formulation success relies on finding cost-effective ingredients with the lowest CMC to ensure rapid surface tension reductions. A new trend is replacing traditional surfactants with biosurfactants, which have a much lower CMC.
One example is Locus Performance Ingredients (Locus PI) line of 100% GMO-free and fermentation-derived sophorolipids (INCI: glycolipids). These biosurfactants have extremely low CMC and are also sulfate-, ethoxylate- and palm oil-free. It makes them a top choice for formulators looking to add sustainable products to their offerings.
The innovation comes from the fermentation technology used to make these 100% bio–based surfactants that are free from petrochemicals and easily customizable. The unique characteristics and low CMC of these biosurfactant ingredients offers multifunctional uses in personal care, household or indusrial applications. It gives formulators new tools in the quest to deliver high performance, clean beauty offerings.
The BioChem renaissance has started and the Locus PI series of sophorolipids is an example of an ingredient trend that is better for consumers, for brands and for the planet.


